18+ Calculate The Molality Of The Following Aqueous Solutions
Of 050 M NaOH in a StyrofoamTM cup calorimeter. Web Quantification of solubility.

Calculate The Molalities Of The Following Aqueous Solutions 12 Points A 122 M Course Hero
Air for example is a solution.

. Have information about the structure and intermolecular forces of the drug. Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Both solutions were initially at 200 C but when the two were mixed the temperature rose to 232 C a Suppose the experiment is repeated in the same calorimeter but this time using 200 mL of 050 M HCl.
Of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or solution. Get accurate and detailed answers to all your questions in free downloadable PDF files. The correct option is c.
Sports drinks such as Gatoraid have combinations of these key electrolytes to help replenish electrolyte loss following a hard workout. 1 L of solution 1000 mL 1000 cm 3. Calculate the mass percentage of the resulting solution.
1329 gcm 3 times 1000 cm 3 1329 g the mass of the entire solution. Calculate the by mass Na2SO4 of the solution. Of 050 M HCI and 1000 ml.
K b for water 0512 K kg mol-1 Delhi 2012 Answer. Eg 5 vv ethanol in water. Drugs with low aqueous solubility often present problems related to their formulation and.
Web Access NCERT Solutions for Class 12 Chemistry on Chapter 2 Solutions. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. Ionic covalent covalent B.
Web A student performing a calorimetry experiment combined 1000 ml. 1 1270g of iodine 2 480g of magnesium. Any of the several ways of expressing concentration of solutions can be used such as the mass volume or amount in moles of the solute for a specific mass volume or mole amount of.
Web b An aqueous solution containing 1248 g of barium chloride in 10 kg of water boils at 3730832 K. 1329 g minus 5714 g 7576 g 07576 kg the mass of water in the. The quantity of solute that is dissolved in a particular quantity of solvent or solution.
This is given below. Solutions are all around us. If the ratio is vv that means such quantity of solvent will be mix with another quantity of solvent then qs.
We have to Calculate the molality of 150 grams of KBr dissolved in 7500 mL pure water. A Molality of KI b Molarity of KI c Mole fraction of KI. Substitute these values into the equation for freezing point depression.
Web JEE Main Mole Concept Previous Year Questions With Solutions. Calculate the molarity of each of the following solutions a 30 g of CoNO 326H 2 O in 43 L of solution b 30 mL of 0-5 M H 2 SO 4 diluted to 500 mL. Web Download these NCERT Solutions for Class 12 Chemistry and prepare well for the Class 12 board exam.
Required in making 25 kg of 025 molal aqueous solution. The molarity of a mixture M mix can be calculated using the following. 025 Molal aqueous solution to urea means that moles of urea.
Web Calculate the molality of each of the following solutions. Ionic ionic covalent C. A sulfuric acid solution containing 5714 g of H 2 SO 4 per liter of solution has a density of 1329 gcm 3Calculate the molality of H 2 SO 4 in this solution.
Calculate the mass of urea NH 2 CONH 2 required in making 25 kg of 025 molal aqueous solution. Ionic metal Classify the following compounds as ionic or covalent. Frac03751000times 500 01875 mole.
Web FloryHuggins solution theory is a lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixingThe result is an equation for the Gibbs free energy change for mixing a polymer with a solventAlthough it makes simplifying assumptions it. I Molality is the number of moles of solute per 1000 g of solvent whereas molarity is the number of moles of solute per 1000 ml of the solution. The solubility of a specific solute in a specific solvent is generally expressed as the concentration of a saturated solution of the two.
So we can calculate the number of moles of sodium acetate in 500 ml. As ΔT b iK b m 10018 100 C i 0512 K kg mol-1 1 m. Web The development of pharmaceutical technology in past years has presented the development of alternative dosage forms for patients who may have difficulty in swallowing of conventional tablets.
Concentration refers to the amount of solute that is dissolved in a solventWe normally think of a solute as a solid that is added to a solvent eg adding table salt to water but the. Determine the vant Hoff factor for trichloroacetic acid. 0375 M aqueous Ans of sodium acetate means 1000 ml of Ans contains 0375 moles of sodium acetate.
The molality of the solution will be molar mass NaCl 585 g mol-1 1 218 m 2 300 m. What mass in grams of NaCl needs to be added to 16 kg of water in order to create a solution with a freezing point of -56 C. An aqueous Na2SO4 solution has a molality of 177 m and a density of 105 gmL.
Web Calculating the concentration of a chemical solution is a basic skill all students of chemistry must develop early in their studies. What is the molarity of the final mixture. Calculate the mass of FeSO 47H 2 O which must be added in.
Important physiological electrolytes include Na K Ca 2 Mg 2 and Cl. Argon has 18 protons and the isotope has 22 neutrons so the mass number of the isotope is 40. We are also given the molar mass of sodium acetate is 820245text g moltexte-1.
M L 1 is the density of 20 aqueous KI determine the following. Volume of formic acid 300 ml Molarity of formic acid 165 M M1 Volume of sodium. Two solutions of a substance non-electrolyte are mixed in the following manner.
Molar mass of BaCl 2 20834 g mol-1 Answer. Web The term electrolyte is used in medicine to mean any of the important ions that are dissolved in aqueous solution in the body. Number of atoms in the following samples of substances is the largest in.
Molality is given as ratio of moles of solute per gm of mass of solvent. Web calculate the moles of solute from the mass and molar mass of the solute and divide by the mass of water in kilograms. Web ii Calculate the freezing point of an aqueous solution contaning 1050 g of MgBr2 in 200 g of water Molar mass of MgBr 2 184 g mol-1 for water 186 K kg mol-1.
How is the molality of a solution different from its molarity. Calculate the degree of dissociation of barium chloride. Many drugs are formulated as solutions or added as powder or solution forms to liquids.
Given for H 2 0 052 K m-1. CBSE Delhi 2011 Answer. 2010 Very Short Answer Type Questions 1 Mark 69.
Web In Section 93 we described various ways of characterizing the concentration of solution molarity M molality m percent concentrations and mole fraction X. The solution has the boiling point of 10018C. Web Given the following reactions calculate ΔH in kJ for the reaction 2 NO2 g 12 O2 g N2O5 g.
A 583 g of H 2 SO 4 in 150 kg of waterthe acid solution used in an automobile battery b 086 g of NaCl in 100 10 2 g of watera solution of sodium chloride for intravenous injection c 4685 g of codeine C 18 H 21 NO 3 in 1255 g of ethanol C 2 H 5 OH. 480 mL of 15 M first solution 520 mL of 12 M second solution. What is the pH of a buffer solution if you dissolve 300 ml of 165M Formic Acid HCO2H and 250 ml A.
Web Study with Quizlet and memorize flashcards containing terms like Carbon dioxide is a _____ compound composed two types of _____ atoms. Web A 100 molal aqueous solution of trichloroacetic acid CCl 3 COOH is heated to its boiling point. Web Last part would be.
Calculate the mass of a non-volatile. Overcome problems arising during preparation of pharmaceutical solutions.

Jee Main Solved Papers Iit Books Pdf Pdf

Chemistry Of Atmospheric Brown Carbon Chemical Reviews

Calculate The Molalities Of The Following Aqueous Solutions 12 Points A 122 M Course Hero
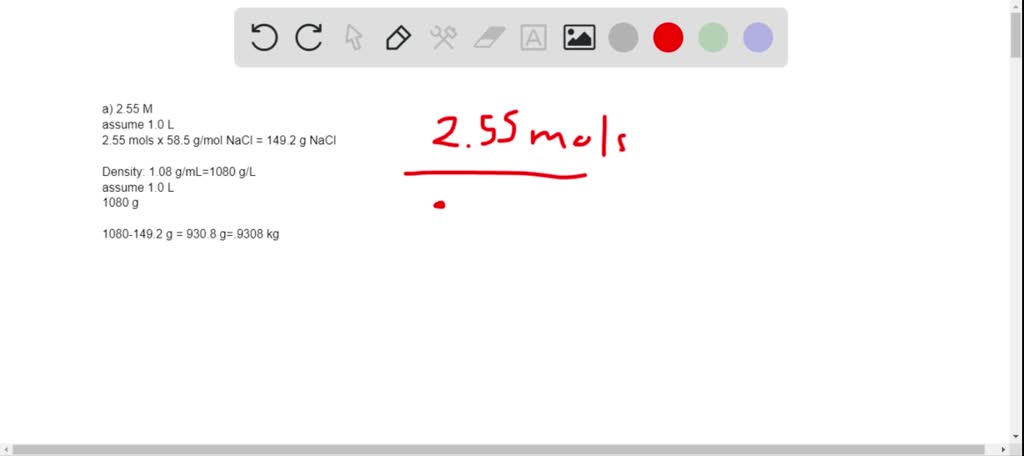
Solved Calculate The Molality Of Each Of The Following Aqueous Solutions A 2 55 M Nacl Solution Density Of Solution 1 08 G Ml B 45 2 Percent By Mass Kbr Solution

Calculate The Molalities Of The Following Aqueous Solutions 12 Points A 122 M Course Hero

Solved Calculate The Molality Of Each Of The Following Aqueous Solutions A 2 50 M Nacl Solution Density Of Solution 1 08 G Ml B 48 2 Percent By Mass Kbr Solution
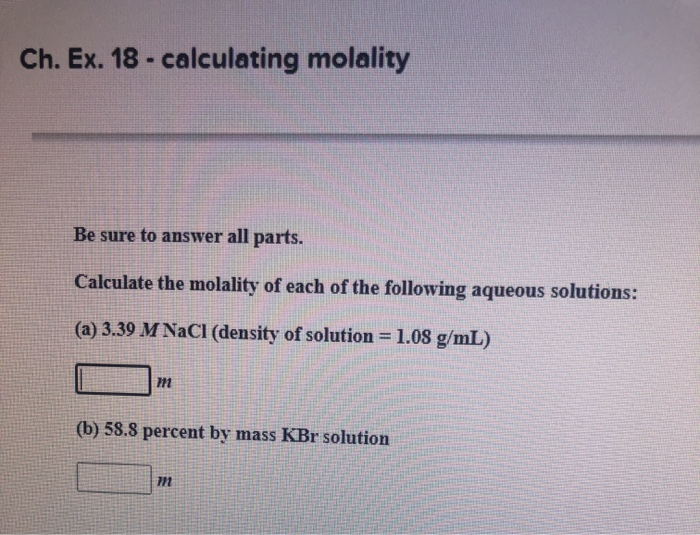
Solved Ch Ex 18 Calculating Molality Be Sure To Answer Chegg Com

What Is Molality Of Nacl In An Aqueous Solution Which Is 4 20 Molar The Density Of The Solution Chemistry Solutions 13559410 Meritnation Com

Molality Chemistry Socratic
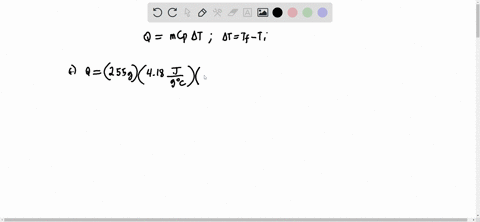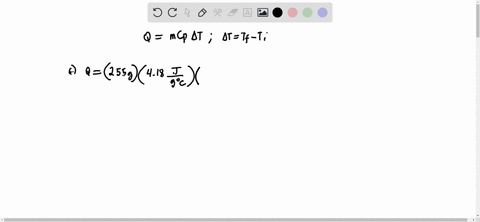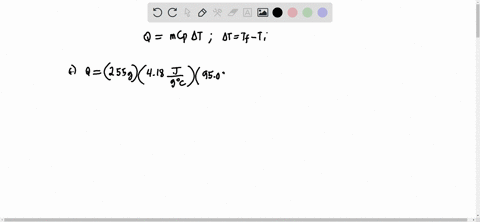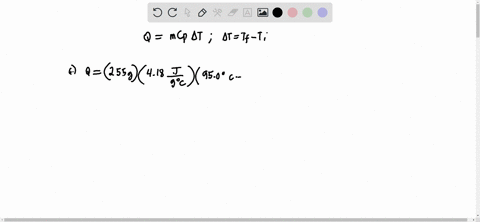
Solved Calculate The Molality Of Each Of The Following Aqueous Solutions A 2 50 M Nacl Solution Density Of Solution 1 08 G Ml B 48 2 Percent By Mass Kbr Solution

Calculate The Molalities Of The Following Aqueous Solutions 12 Points A 122 M Course Hero

Solved I Need The Molality Of Solution Containing Chegg Com

Calculate The Molarity And Molality Of The Following Solution Nacl Solution Prepared By Dissolving 35 G Of It In 250 Chemistry Some Basic Concepts Of Chemistry 13571424 Meritnation Com

Clinical Chemistry Powerpoint Slides

Solved Calculate The Molality Of Each Of The Following Aqueous Solutions A 2 55 M Nacl Solution Density Of Solution 1 08 G Ml B 45 2 Percent By Mass Kbr Solution

Solved 6 1 16 Determine The Molality Of Each Of The Chegg Com

Calculate The Molality Of Aqueous Solution Of An Unknown Solute If Mole Fraction Of Water Youtube